Tuning primary and secondary coordination spheres of ruthenium complexes for the homogeneous water oxidation reaction: a perspective from catalytic activity and overpotential
Abstract
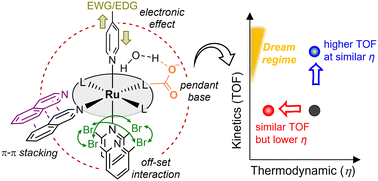
Molecular ruthenium (Ru) complexes derived from the Ru blue dimer complex have been extensively studied for water oxidation. For example, monomeric Ru catalysts of polypyridyl-type ligands, such as 2,2-bipyridine-6,6-dicarboxylate (bda), are a representative family of Ru-based water oxidation catalysts. Tremendous efforts have been made in the engineering of primary and secondary coordination spheres to promote the activity of Ru catalysts. Nonetheless, cross-comparison of the Ru catalysts used among different studies is challenging because the reaction conditions are dissimilar. In this comment, the performance of single-site Ru catalysts bearing polypyridyl-type ligands has been investigated in terms of their kinetic and thermodynamic behavior. The analysis parameters include normalized descriptors, turnover frequency (TOF), and overpotential (η). The catalyst performance is further compared and analyzed on the basis of the relationship between the TOF and η. In addition, the impact of the primary and secondary coordination spheres on the reaction mechanism has been discussed. We expect that these analyses will open an avenue for exploring the next generation of molecular Ru-based water-oxidation catalysts.


 Please wait while we load your content...
Please wait while we load your content...