The copper-catalyzed oxidative radical process of site selective C–N bond cleavage in twisted amides: batch and continuous-flow chemistry†
Abstract
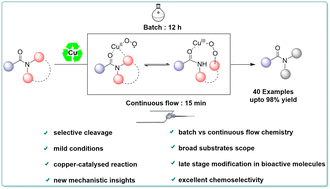
Due to the significance of amide bonds, it is crucial to develop a new catalytic strategy to produce amides by selective C–N bond cleavage, one of the most potent and rapid processes in chemical synthesis. This work represents the first example of copper-superoxo radical activation in twisted amides which mediates the aerobic oxidative process. Herein, we describe a mild, simple, chemoselective and copper-catalyzed method to synthesize primary amides from readily available and bench stable crystalline solid N-acyl glutarimide. Pertinently, this copper catalytic transformation permits formation of a wide range of amides in batch and continuous-flow conditions effectively. Moreover, the reaction shows excellent functional group tolerance in high yields and is applicable for wide substrate scope including late-stage functionalization of complicated APIs. While using this copper-catalytic C–N bond cleavage in applications, gram-scale primary amide and various important scaffolds were successfully synthesized. We further present mechanistic and UV-visible spectral studies that outline the copper reactive oxygen species involved in the reaction mechanism for selective C–N bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...