A computational investigation of the decomposition of acetic acid in H-SSZ-13 and its role in the initiation of the MTO process†
Abstract
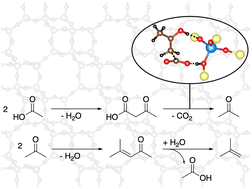
The zeolite-catalyzed reaction of acetic acid is important in the direct utilization of biomass and also plays a role in the reactivity of oxygenates in the methanol-to-olefins (MTO) process. The conversion of acetic acid to acetone involves the coupling of two acetic acid molecules to a β-ketoacid (3-oxobutanoic acid) in a first step, which can then be decarboxylated to acetone. Further possible reactions include the aldol self-condensation of acetone to mesityl oxide, which can subsequently decompose to isobutene and acetic acid. We investigate reaction pathways in H-SSZ-13 from acetic acid to isobutene using periodic density functional theory in combination with DLPNO-CCSD(T) calculations on cluster models. For the formation of 3-oxobutanoic acid, we propose a mechanism including the coupling of a ketene and a surface acetate with free energy barriers of 197 kJ mol−1 at most. Further free energy barriers leading to isobutene are lower. Studying reaction kinetics with a batch reactor model at 400 °C, we find fast conversion of acetic acid to acetone, which is a stable intermediate. The further reaction to isobutene is slower. In addition, we perform kinetic simulations which predict a minor relevance of these reactions for the initiation of the MTO process.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...