Effect of alkali metal addition on catalytic performance of Ag/ZrO2/SBA-16 catalyst for single-step conversion of ethanol to butadiene†
Abstract
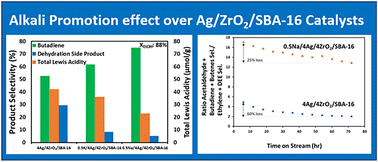
This paper describes how adding Na and K to a 4Ag/4ZrO2/SBA-16 catalyst enhances catalytic performance for single-bed conversion of ethanol to butadiene. While adding Na and K leads to a slight decrease in conversion (i.e., ∼10% loss), the production of desired butadiene is significantly increased with up to 50% improvement in productivity for the 4Ag/4ZrO2/SBA-16 catalyst promoted with 0.5% Na. The reasons for this improvement are a beneficial decrease in Lewis acid site concentration and higher Ag dispersion when Na or K are added, which results in decreased activity involving ethanol dehydration to ethylene and diethyl ether. A remarkable butadiene selectivity of 75% was achieved while maintaining high conversion (i.e., 90%) with 0.5Na/4Ag/4ZrO2/SBA-16 catalyst. A 72-hour catalyst lifetime study shows that because of higher coke formation from polymerization of desired butadiene, catalyst deactivation occurs more rapidly with the 0.5Na/4Ag/4ZrO2/SBA-16 (55% loss in conversion) than with 4Ag/4ZrO2/SBA-16 (45% loss in conversion). However, this does not alter the advantageous effect of Na addition because the butadiene yield remained higher throughout the study period for 0.5Na/4Ag/4ZrO2/SBA-16. A key finding is that during the reaction, Na limits sintering of Ag particles and promotes selective coking of the acid sites responsible for ethylene and diethyl ether formation.


 Please wait while we load your content...
Please wait while we load your content...