Accelerated H2 activation over Pt/M-ZrO2 for the reductive amination of levulinic acid esters under benign conditions†
Abstract
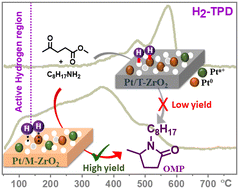
Catalytic reductive amination of biomass-derived levulinic acid and its esters to pyrrolidones, having numerous applications in the pharmaceutical and agrochemical fields, is an attractive approach. A robust Pt-impregnated monoclinic ZrO2 (Pt/M-ZrO2) catalyst is developed for the reductive amination of methyl levulinate (MLevu) with octyl amine (OA) in the presence of H2, obtaining a quantitative yield of N-octyl-5-methyl-2-pyrrolidones (OMP) at ambient temperature. Adversely, Pt-impregnated tetragonal ZrO2 (Pt/T-ZrO2) displays an 18-fold lower activity than Pt/M-ZrO2 in terms of turnover frequency at similar conversion. Transmission electron microscopy with energy dispersive X-ray (TEM-EDX) spectroscopy suggests higher Pt dispersion in Pt/M-ZrO2, having a 15-fold higher surface area than Pt/T-ZrO2, as evidenced by N2-sorption analysis. Pt/M-ZrO2 contains 15 and 2-fold more acidic and basic sites than Pt/T-ZrO2, respectively, which helps to activate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the formed intermediate and H2, thus facilitates hydrogenation to form OMP selectively. Further, the poisoning studies reveal that both acidic and basic sites in Pt/M-ZrO2 play a vital role in enhancing the yield of OMP, indicating the formation of a frustrated Lewis pair (FLP) that facilitates the reaction at room temperature. Moreover, H2-temperature programmed desorption (H2-TPD) evidently supports that Pt/M-ZrO2 possesses a low-temperature desorption peak (50–100 °C), which is absent in the case of Pt/T-ZrO2, indicating high H2 activation and hydrogenation ability at room temperature which significantly contributes to hydrogenation of the formed imine intermediate to OMP. Further, Pt/M-ZrO2 is recyclable for at least three times with no significant changes in the conversion and yield.
N bond of the formed intermediate and H2, thus facilitates hydrogenation to form OMP selectively. Further, the poisoning studies reveal that both acidic and basic sites in Pt/M-ZrO2 play a vital role in enhancing the yield of OMP, indicating the formation of a frustrated Lewis pair (FLP) that facilitates the reaction at room temperature. Moreover, H2-temperature programmed desorption (H2-TPD) evidently supports that Pt/M-ZrO2 possesses a low-temperature desorption peak (50–100 °C), which is absent in the case of Pt/T-ZrO2, indicating high H2 activation and hydrogenation ability at room temperature which significantly contributes to hydrogenation of the formed imine intermediate to OMP. Further, Pt/M-ZrO2 is recyclable for at least three times with no significant changes in the conversion and yield.


 Please wait while we load your content...
Please wait while we load your content...