Topology of electrostatic potential and electron density reveals a covalent to non-covalent carbon–carbon bond continuum†
Abstract
The covalent and non-covalent nature of carbon–carbon (CC) interactions in a wide range of molecular systems can be characterized using various methods, including the analysis of molecular electrostatic potential (MESP), represented as V(r), and the molecular electron density (MED), represented as ρ(r). These techniques provide valuable insights into the bonding between carbon atoms in different molecular environments. By uncovering a fundamental exponential relationship between the distance of the CC bond and the highest eigenvalue (λv1) of V(r) at the bond critical point (BCP), this study establishes the continuum model for all types of CC interactions, including transition states. The continuum model is further delineated into three distinct regions, namely covalent, borderline cases, and non-covalent, based on the gradient, 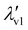 , with the bond distance of the CC interaction. For covalent interactions, this parameter exhibits a more negative value than −5.0 a.u. Å−1, while for non-covalent interactions, it is less negative than −1.0 a.u. Å−1. Borderline cases, which encompass transition state structures, fall within the range of −1.0 to −5.0 a.u. Å−1. Furthermore, this study expands upon Popelier's analysis of the Laplacian of the MED, denoted as ∇2ρ, to encompass the entire spectrum of covalent, non-covalent, and borderline cases of CC interactions. Therefore, the present study presents compelling evidence supporting the concept of a continuum model for CC bonds in chemistry. Additionally, this continuum model is further explored within the context of C–N, C–O, C–S, N–N, O–O, and S–S interactions, albeit with a limited dataset.
, with the bond distance of the CC interaction. For covalent interactions, this parameter exhibits a more negative value than −5.0 a.u. Å−1, while for non-covalent interactions, it is less negative than −1.0 a.u. Å−1. Borderline cases, which encompass transition state structures, fall within the range of −1.0 to −5.0 a.u. Å−1. Furthermore, this study expands upon Popelier's analysis of the Laplacian of the MED, denoted as ∇2ρ, to encompass the entire spectrum of covalent, non-covalent, and borderline cases of CC interactions. Therefore, the present study presents compelling evidence supporting the concept of a continuum model for CC bonds in chemistry. Additionally, this continuum model is further explored within the context of C–N, C–O, C–S, N–N, O–O, and S–S interactions, albeit with a limited dataset.

- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...