Mechanism of formation and ion mobility separation of protomers and deprotomers of diaminobenzoic acids and aminophthalic acids†
Abstract
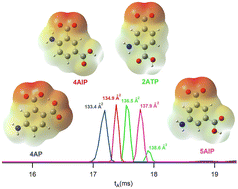
Aminobenzoic acids are well-established candidates for understanding the formation of isomeric ions in positive mode electrospray ionization as they yield both N- and O-protomers (prototropic isomers) at the amine and carbonyl sites, respectively. In the present work, a combination of ion mobility-mass spectrometry and density functional theory calculations to determine the protonation and deprotonation behaviour of four diamino benzoic acid and four aminophthalic acid isomers is presented. The additional COOH group on the ring of aminophthalic acids provides experimental evidence regarding the mechanism of intramolecular NH3+ → O proton transfer, which has been the subject of debate in recent years. To determine the proton acceptor O atom, ion mobility spectra of the fragments of protomers were used as a new method for the confidential assignment of the O-protomer structure, confirming only short-distance intramolecular NH3+ → O proton transfer. Additionally, the substitution pattern both influences the basicity of the protonation sites and enables these molecules to form internal hydrogen bonds with the protonated or deprotonated sites. The formation of the hydrogen bonds in the deprotonated aminophthalic acids changed the charge distribution and subsequently their ion mobility-derived collision cross sections in nitrogen (CCSN2) leading to separation of the four isomers studied. Finally, an interesting effect of the substitution pattern was observed as a synergistic electron-donating effect of the amine groups of 3,5-diaminobenzoic acid on enhancing the basicity of the carbon atom C2 of the ring and previously unreported formation of a C-protomer within aminobenzoic acid systems.


 Please wait while we load your content...
Please wait while we load your content...