Room temperature epoxidation of ethylene over delafossite-based AgNiO2 nanoparticles†
Abstract
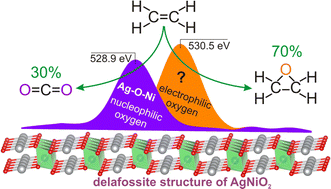
A mixed oxide of silver and nickel AgNiO2 was obtained via co-precipitation in alkaline medium. This oxide demonstrates room temperature activity in the reaction of ethylene epoxidation with a high selectivity (up to 70%). Using the PDF method, it was found that the initial structure of AgNiO2 contains stacking faults and silver vacancies, which cause the nonstoichiometry of the oxide (Ag/Ni < 1). It has been established that on the initial surface of AgNiO2 oxide, silver state can be considered as an intermediate between Ag2O and Ag0 (i.e. Agδ+-like), while nickel is characterized by signs of a deeply oxidized state (Ni3+-like). The interaction of AgNiO2 with C2H4 at room temperature leads to the simultaneous removal of two oxygen species with Eb(O 1s) = 529.0 eV and 530.5 eV considered as nucleophilic and electrophilic oxygen states, respectively. Nucleophilic oxygen was attributed to the lattice oxygen (Ag–O–Ni), while the electrophilic species with epoxidation activity was associated with the weakly bound oxygen stabilized on the surface. According to the TPR-C2H4 data, a large number of weakly bound oxygen species were found on the pristine AgNiO2 surface. The removal of such species at room temperature didn’t result in noticeable structural transformation of delafossite. As the temperature of ethylene oxidation over AgNiO2 increased, the appearance of Ag0 particles was first observed below 200 °C followed by the complete destruction of the delafossite structure at higher temperatures.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...