Revisit of the plasmon-mediated chemical transformation of para-aminothiophenol†
Abstract
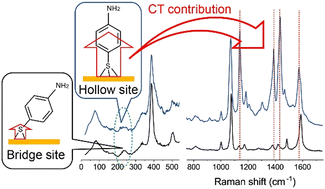
Fingerprint Raman features of para-aminothiophenol (pATP) in surface-enhanced Raman scattering (SERS) spectra have been widely used to measure plasmon-driven catalytic activities because the appearance of characteristic spectral features is purported to be due to plasmon-induced chemical transformation of pATP to trans-p,p′-dimercaptoazobenzene (trans-DMAB). Here, we present a thorough comparison of SERS spectra for pATP and trans-DMAB in the extended range of frequencies covering group vibrations, skeletal vibrations, and external vibrations under various conditions. Although the fingerprint vibration modes of pATP could be almost mistaken with those of trans-DMAB, the low-frequency vibrations revealed distinct differences between pATP and DMAB. Photo-induced spectral changes of pATP in the fingerprint region were explained well by photo-thermal variation of the Au–S bond configuration, which affects the degree of the metal-to-molecule charge transfer resonance. This finding indicates that a large number of reports in the field of plasmon-mediated photochemistry must be reconsidered.


 Please wait while we load your content...
Please wait while we load your content...