A new green-to-blue upconversion system with efficient photoredox catalytic properties†
Abstract
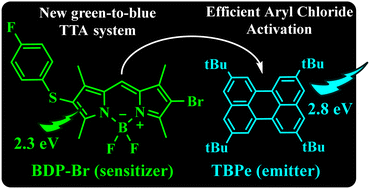
The design and development of new triplet–triplet annihilation upconversion (TTA-UC) systems combining triplet sensitizers with acceptor compounds have attracted considerable interest. In this vein, sensitizers made from purely organic dyes rather than transition-metal complexes appear to be more convenient from an environmental point of view. BODIPYs are a very well-known class of dyes with applications in a widespread range of scientific areas. Owing to the versatility of BODIPYs, we present herein a new asymmetric BODIPY with excellent photophysical properties to be used as an appropriate sensitizer in a bimolecular TTA-UC system. Detailed spectroscopic measurements demonstrated the ability of this new design to sensitize TTA-UC by combination with a suitable acceptor such as 2,5,8,11-tetra-tert-butylperylene (TBPe), allowing a successful conversion of green to blue light. The singlet-excited TBPe so obtained is capable of activating aryl chlorides reductively which initiated the functionalization of N-methylpyrrole (Meerwein-type arylation) and formation of both substituted triarylethylenes (Mizoroki–Heck reaction) and heteroarene phosphonates (photo-Arbuzov reaction). Product yields reveal that our TTA-UC system behaved as a highly efficient photocatalytic entity.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...