Synergic photoprotection of phenolic compounds present in tomato fruit cuticle: a spectroscopic investigation in solution†
Abstract
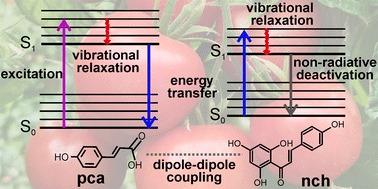
Coumaric acids and flavonoids play pivotal roles in protecting plants against ultraviolet radiation (UVR) exposure. In this work, we focus our photoprotection studies on p-coumaric acid and the flavonoid naringenin chalcone. Photoprotection is well-understood in p-coumaric acid; in contrast, information surrounding photoprotection in naringenin chalcone is lacking. Additionally, and vitally, how these two species work in unison to provide photoprotection across the UV-B and UV-A is unknown. Herein, we employ transient absorption spectroscopy together with steady-state irradiation studies to unravel the photoprotection mechanism of a solution of p-coumaric acid and naringenin chalcone. We find that the excited state dynamics of p-coumaric acid are significantly altered in the presence of naringenin chalcone. This finding concurs with quenching of the p-coumaric acid fluorescence with increasing concentration of naringenin chalcone. We propose a Förster energy transfer mechanism is operative via the formation of dipole–dipole interactions between p-coumaric acid and naringenin chalcone. To our knowledge, this is the first demonstration in plants of a synergic effect between two classes of phenolics to bypass the potentially damaging effects of UVR.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...