Proton-donating and chemistry-dependent buffering capability of amino acids for the hydrogen evolution reaction†
Abstract
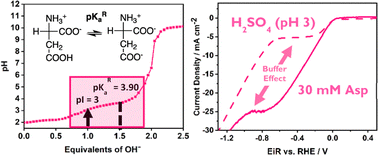
The hydrogen evolution reaction (HER) has been widely demonstrated to have a strong dependence on pH and on the source of protons, where a clear kinetic advantage arises in acidic conditions over near-neutral and alkaline conditions due to the switch in reactant from H3O+ to H2O. Playing on the acid/base chemistry of aqueous systems can avoid the kinetic frailties. For example, buffer systems can be used to maintain proton concentration at intermediate pH, driving H3O+ reduction over H2O. In light of this, we examine the influence of amino acids on HER kinetics at platinum surfaces using rotating disk electrodes. We demonstrate that aspartic acid (Asp) and glutamic acid (Glu) can act not only as proton donors, but also have sufficient buffering action to sustain H3O+ reduction even at large current density. Comparing with histidine (His) and serine (Ser), we reveal that the buffering capacity of amino acids occurs due to the proximity of their isoelectric point (pI) and their buffering pKa. This study further exemplifies HER's dependence on pH and pKa and that amino acids can be used to probe this relationship.


 Please wait while we load your content...
Please wait while we load your content...