Elucidating the complete oxidation mechanism of betanidin in an aqueous solution†
Abstract
An important point to take advantage of the use of antioxidants in industrial applications in a more efficient way is to know in depth their oxidation mechanism. This is not always a simple task and requires an in-depth study that is often insufficient to precisely describe all the structures and processes involved. This is the case of betanidin, a natural pigment employed in the drug, food, and cosmetic industries. In the present work, we seek to unravel the complete oxidation mechanism of betanidin with the use of computational techniques, supported by experimental data. For this aim, the pKas and oxidation potentials 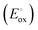 of the reactions involved at different pHs were analyzed using density functional theory (DFT) with the B3LYP/6-31+G(d,p)/SMD approach. Moreover, the decomposition mechanism of the intermediate products (decarboxylation reactions) was studied deeply. The analysis of DFT results allowed the proposal of a tentative mechanism that was put to test using the digital simulations of cyclic voltammetry by comparing the results of these simulations with an experimental case. Based on the rigorous experimental analysis, DFT, and simulations of cyclic voltammetry, the complete mechanism of the oxidation of betanidin in an aqueous medium was proposed. The dimerization of the oxidation products was also considered to explain the voltammetric response of betanidin.
of the reactions involved at different pHs were analyzed using density functional theory (DFT) with the B3LYP/6-31+G(d,p)/SMD approach. Moreover, the decomposition mechanism of the intermediate products (decarboxylation reactions) was studied deeply. The analysis of DFT results allowed the proposal of a tentative mechanism that was put to test using the digital simulations of cyclic voltammetry by comparing the results of these simulations with an experimental case. Based on the rigorous experimental analysis, DFT, and simulations of cyclic voltammetry, the complete mechanism of the oxidation of betanidin in an aqueous medium was proposed. The dimerization of the oxidation products was also considered to explain the voltammetric response of betanidin.



 Please wait while we load your content...
Please wait while we load your content...