Effects of oxygen pressure on the morphology and surface energetics of β-PbO2: insight from DFT calculations†
Abstract
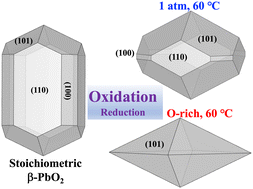
For over a century, lead dioxide (PbO2) has been investigated in lead–acid batteries and extensively utilized in a variety of applications. Identifying the surface properties and equilibrium morphology of β-PbO2 (rutile phase) particles is mandatory for industrial utilization and surface engineering. Using density-functional calculations within the generalized gradient approximation revised for solids (PBEsol), we investigate a variety of surface properties of β-PbO2. The surface energies of low-Miller-index stoichiometric surfaces are firstly determined, and the (110) surface is found to be the most thermodynamically stable. The relative energetics of these surfaces are represented by a Wulff construction which shows an acicular shape, mostly dominated by the (110) and (100) surfaces. Besides, we investigate the surface chemistry of β-PbO2 under reduction and oxidation conditions as a function of oxygen pressure, finding that most surfaces except for (100) and (110) are likely to be oxidized. Under oxygen pressure at 1 atm and oxygen-rich limit, the (101) surface is the most thermodynamically stable, dominating the Wulff construction with pyramidal shapes. Our results indicate that the growth conditions that cause non-stoichiometry of the surface could modify the equilibrium Wulff shape of β-PbO2. Our predicted Wulff shapes and dominant facets agree with the experimental results in which the pyramidal shape of the β-PbO2 grains has often been observed with the (101) preferred orientation.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...