Softening by charging: how collective modes of ionic association in concentrated redoxmer/electrolyte solutions define the structural and dynamic properties in different states of charge†
Abstract
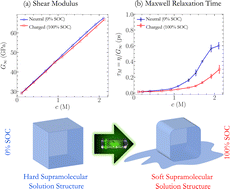
Understanding the physical and chemical processes occurring in concentrated electrolyte solutions is required to achieve redox flow batteries with high energy density. Highly concentrated electrolyte solutions are often studied in which collective crowded interactions between molecules and ions become predominant. Herein, experimental and computational methods were used to examine non-aqueous electrolyte solutions in two different states of charge as a function of redoxmer concentration. As the latter increases and the ionic association strengthens, the electric conductivity passes through a maximum and the solution increasingly gels, which is seen through a rapid non-linear increase in viscosity. We establish that the structural rigidity of ionic networks is closely connected with this loss of fluidity and show that charging generally yields softer ionic assemblies with weaker attractive forces and improved dynamical properties.


 Please wait while we load your content...
Please wait while we load your content...