π–π stacking in the polymorphism of 2-(naphthalenylamino)-nicotinic acids and a comparison with their analogues†
Abstract
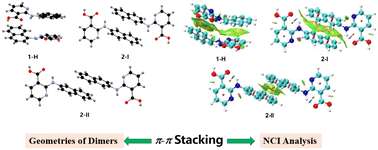
Substituent size and isomerization play an important role in the polymorphism of 2-(naphthalen-n-ylamino)-benzoic acids (n = 1, 2; 2-NBAs) as each of the two previously investigated 2-NBAs was found to be polymorphic, while their parent compound fenamic acid (FA) has only one form discovered. 2-(Naphthalen-1-ylamino)-nicotinic acid (1) and 2-(naphthalen-2-ylamino)-nicotinic acid (2) (2-NNA) are isosteric compounds of 2-NBAs, and 2-(2,3-dimethyl-phenylamino)-nicotinic acid (3) and 2-(3,4-dimethyl-phenylamino)-nicotinic acid (4) are 2-NNA analogues with similar substitution patterns. All four compounds are clonixin analogues. The polymorphism of these four compounds was investigated. One solvent-free form (1-A) and one hydrate (1-H) were discovered for 1, three forms (2-I, 2-II, and 2-III) were obtained for 2, and one form was found for 3 and 4. The four systems were characterized by single-crystal XRD (SCXRD) and powder XRD (PXRD), and structural properties were compared among the crystals of the four compounds, their isosteres, and other related analogues. The phase behavior of the four systems was studied with differential scanning calorimetry (DSC). Thermogravimetric analysis (TGA) was used to investigate the dehydration of 1-H. Computational studies such as stable conformer search, π–π interaction energy calculation, and non-covalent interaction (NCI) analysis were performed to shed light on the rise of polymorphism, and the relative contribution of various intermolecular interactions to the overall stability of the crystals. The study confirmed the effect of substituent size, thus the consequent π–π interactions, on the hydrate formation of compound 1 and polymorphism of compound 2.


 Please wait while we load your content...
Please wait while we load your content...