Graphite/diamond transformation mechanism under the action of an iron-based catalyst
Abstract
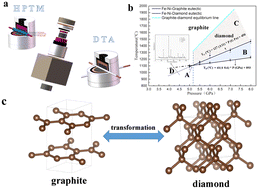
Although it has been nearly 70 years since iron-based catalysts were used to synthesize diamond from graphite at high pressure and high temperature (HPHT), the graphite to diamond transformation mechanism in this process is still a mystery. It is found that the formation of a eutectic melt between carbon and an iron-based catalyst plays a critical role in the graphite to diamond transformation at HPHT. In this work, the eutectic temperature of Fe–Ni–C (diamond and graphite) was determined using the in situ high-pressure temperature measurement (HPTM) and the high-pressure differential thermal analysis (HPDTA) method up to 8 GPa in a large-volume cubic press. The experimental results of XRD, Raman, SEM and XPS indicate that the transformation of graphite to diamond occurs in the Fe–Ni–C eutectic melt: the C–C bonds in graphite are broken in the eutectic melt, and the free carbon atoms in the melt form sp3 bond states in the thermodynamically stable region of diamond. In this process, the role of iron-based catalysts is to reduce the environmental conditions, in which the C–C bonds in graphite are destroyed, and to make the carbon atoms that are separated from the sp2 bond state form the sp3 bond state in the diamond P–T phase stable region.


 Please wait while we load your content...
Please wait while we load your content...