Polymorphism in carboxamide compounds with high-Z′ crystal structures†
Abstract
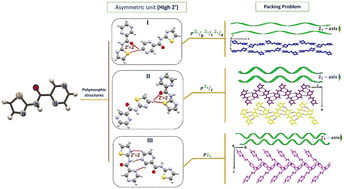
In the present study, a carboxamide compound (N-(1,3-thiazol-2-yl)-2-pyrazine) (L) was designed and synthesized by the reaction of 2-aminothiazol with 2-pyrazine carboxylic acid in a solution-based procedure. L was found to exist in three high Z′ (Z′ = 2) polymorphic structures (I, II, and III). The structural features of three polymorphs were investigated experimentally and theoretically in detail. In order to study non-covalent interaction energy, theoretical studies of the binding energies for all the molecule pairs (dimers) were computed on the polymorphs. The C–H⋯O, C–H⋯N, N–H⋯N, and π-stacking interactions have an important role in the aggregation of molecular structures in the titled polymorphs. The results show that hydrogen bond interactions have a key role to stabilize the symmetry independent (SI) motifs in the high Z′ polymorphs. Interestingly, the 21-axis which was previously reported as one of the reasons for high Z′, was presented in the structure of all of the obtained polymorphs. Phase stability of the mentioned polymorphic system was studied via lattice energy and crystal density. Analysis of these high Z′ polymorphs suggests that the order of stability for these polymorphs can be listed as II > III > I.


 Please wait while we load your content...
Please wait while we load your content...