Phosphorus derivatives of mesoionic carbenes: synthesis and characterization of triazaphosphole-5-ylidene → BF3 adducts†‡
Abstract
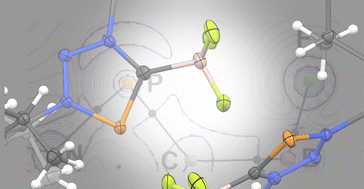
Trimethylsilyl-substituted triazaphospholes were synthesized by a [3+2] cycloaddition reaction between organic azides and (CH3)3Si–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P. In an attempt to isolate their N-alkylated products, the formation of BF3 adducts of unprecedented triazaphosphol-5-ylidenes was found. The nature of the carboncarbene–boron bond was investigated within the DFT framework, revealing a strong donation of electrons from the carbene carbon atom to the boron atom combined with weak back-bonding.
P. In an attempt to isolate their N-alkylated products, the formation of BF3 adducts of unprecedented triazaphosphol-5-ylidenes was found. The nature of the carboncarbene–boron bond was investigated within the DFT framework, revealing a strong donation of electrons from the carbene carbon atom to the boron atom combined with weak back-bonding.
- This article is part of the themed collection: Chemical Communications HOT Articles 2023


 Please wait while we load your content...
Please wait while we load your content...