Light-controlled pKa value of chiral Brønsted acid catalysts in enantioselective aza-Friedel–Crafts reaction†
Abstract
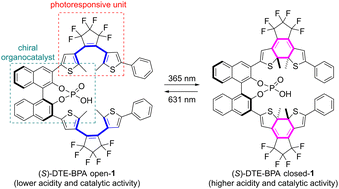
Bis(dithienylethene)-based BINOL-derived phosphoric acid (DTE-BPA) has been developed as a light-controlled chiral organocatalyst for the first time. The photoinduced modulation of the reactivity and selectivity via the open/close isomerization of the DTE scaffold led to superior light-controlled ability in the enantioselective aza-Friedel–Crafts reaction of aldimines with indoles. DFT studies showed that photoisomerization is accompanied by a shift of 1.1 pKa units between the open and closed isomers.


 Please wait while we load your content...
Please wait while we load your content...