Nanotubule inclusion in the channels formed by a six-fold interpenetrated, triperiodic framework†
Abstract
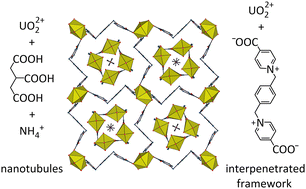
When reacted together with uranyl ions under solvo-hydrothermal conditions, a bis(pyridiniumcarboxylate) zwitterion (L) and tricarballylic acid (H3tca) give the complex [NH4]2[UO2(L)2][UO2(tca)]4·2H2O (1). The two ligands are segregated into different units, an anionic nanotubule for tca3− and a six-fold interpenetrated cationic framework with lvt topology for L. The entangled framework defines large channels which contain the square-profile nanotubules. Complex 1 has a photoluminescence quantum yield of 19% and its emission spectrum shows the superposition of the signals due to the two independent species.


 Please wait while we load your content...
Please wait while we load your content...