Self-assembling materials functionalizing bio-interfaces of phospholipid membranes and extracellular matrices
Abstract
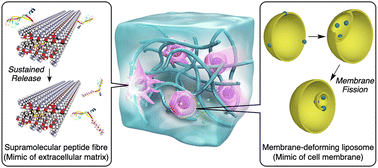
This Feature Article focuses on recent studies on the development of self-assembling materials that mimic and control dynamic bio-interfaces. Extracellular matrix (ECM) is a fundamental tissue at the cellular interface constructed by networks of fibrous proteins, which regulates a variety of cellular activities. Reconstruction of ECM has been demonstrated by self-assembling peptides. By combining the dynamic properties of the self-assembling peptides conjugated with full-length proteins, peptide-based supramolecular materials enable neuronal migration and regeneration of injured neural tissue. The phospholipid bilayer is the main component of the cell membrane. The morphology and deformation of the phospholipid bilayer relate directly to dynamic interfacial functions. Stabilization of the phospholipid nanosheet structure has been demonstrated by self-assembling peptides, and the stabilized bicelle is functional for extended blood circulation. By using a photo-responsive synthetic surfactant showing a mechanical opening/closing motion, endocytosis-like outside-in membrane deformation is triggered. The outside-in deformation allows for efficient encapsulation of micrometer-size substances such as phage viruses into the liposomes, and the encapsulated viruses can be delivered to multiple organs in a living body via blood administration. These supramolecular approaches to mimicking and controlling bio-interfaces present powerful ways to develop unprecedented regenerative medicines and drug delivery systems.
- This article is part of the themed collection: 2023 Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...