Visible light-mediated ring-ablative functionalization of oxazoles: oxidative azidation and demethylative amination†‡
Abstract
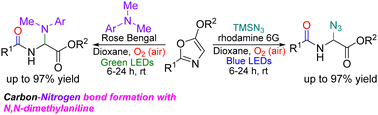
Photochemical functionalization of alkoxyoxazoles with trimethylsilyl azide and N,N-dimethylanilines is described. These C–N bond forming reactions proceed with concomitant oxidative ring-opening assisted by organic dyes as photocatalyst and molecular oxygen, providing access to novel chemical space. Unusual demethylative C–N bond formation in the case of N,N-dimethylanilines establishes a new reactivity pattern for these precursors.


 Please wait while we load your content...
Please wait while we load your content...