A photoresponsive gold catalyst based on azobenzene-functionalized NHC ligands†
Abstract
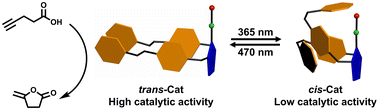
An azobenzene-bearing N-heterocyclic carbene-based gold catalyst is reported of which the reactivity in a cyclization reaction depends on the isomeric state of the azobenzene. The configurations of the catalyst can be reversibly switched by light and are stable during the reaction, effectively leading to a switchable catalyst system.


 Please wait while we load your content...
Please wait while we load your content...