Convergent paired electrolysis for zinc-mediated diastereoselective cinnamylation of α-amino esters†
Abstract
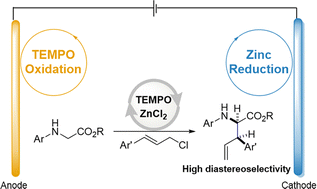
A paired electrochemical method is presented for the one-pot synthesis of γ,δ-unsaturated α-amino esters. The method involves the in situ generation of organozinc reagents through zinc chloride reduction on the nickel cathode and the TEMPO-mediated oxidation of amino esters on the carbon anode. The presence of an ester moiety in the amine substrate was found to be crucial for achieving high diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...