Enantioselective reductive allylic alkylation enabled by dual photoredox/palladium catalysis†
Abstract
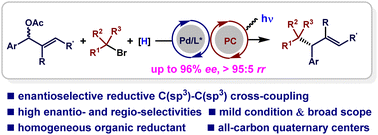
A dual photoredox/palladium catalyzed regio- and enantioselective reductive cross-coupling of allylic acetates with tertiary/secondary alkyl bromides has been achieved, and Hantzsch ester is used as a homogeneous organic reductant. This straightforward protocol enables the stereoselective construction of C(sp3)–C(sp3) bonds under mild reaction conditions. Mechanistic studies suggest that this reaction involves radical pathways and a chiral Pd complex enables the control of the regio- and enantioselectivities.
- This article is part of the themed collections: Chemical Communications HOT Articles 2023 and Photofunctional Materials and Transformations


 Please wait while we load your content...
Please wait while we load your content...