A 1,3-boron shift reaction of homoallenylboronates to synthesise 2-boryl-1,3-dienes†
Abstract
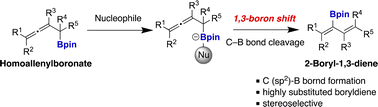
We describe a 1,3-boron shift-type reaction of homoallenylboronates at the center (sp) carbon in allenes to afford 2-boryl-1,3-dienes with a variety of substituents. Notably, this reaction occurs in situ with allenylboronates in the presence of carbamate and a small excess of sec-BuLi, and it is not necessary to isolate the unstable homoallenylboronates.


 Please wait while we load your content...
Please wait while we load your content...