Ring-fused hexahydro-1,2,4,5-tetrazines: synthesis, structure, and mechanistic studies on isolable rotational isomers†
Abstract
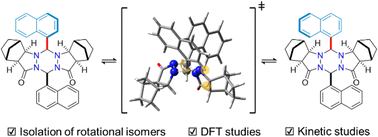
We designed conformationally stable rotational isomers around the C(sp2)–C(sp3) axis at the C3-position of hexahydro-1,2,4,5-tetrazines. Isolation of each rotamer by silica gel column chromatography was successfully achieved at room temperature. The proposed isomerization mechanism of the rotamers was supported by NMR kinetic studies.


 Please wait while we load your content...
Please wait while we load your content...