Decoupled alkaline water electrolysis by a K0.5MnO2-Ti mediator via K-ion insertion/extraction†
Abstract
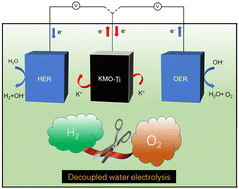
Conventional one-step water electrolyzers generate H2 accompanied by O2 evolution, and may cause gas mixing and high cell voltage inputs. Herein, using the potassium ion battery material of K0.5MnO2-Ti as a mediator, we effectively decoupled the H2 and O2 evolution of alkaline water electrolysis temporally, thereby achieving a membrane-free pathway for H2 production. As a mediator electrode for charge storage, the K0.5MnO2-Ti exhibited a stable capacity of 100 mA h g−1 at 0.1 A g−1 owing to the reversible K-ion insertion/extraction mechanism. The decoupled water electrolysis device exhibited the step voltages for hydrogen and oxygen production of 1.02 and 0.57 V at 5 mA, respectively. A nearly unity Faradaic efficiency and sustained production of pure H2 has been demonstrated at a constant current density. We anticipate that this mediator demonstrated here may provide a route for the practical application of the decoupling strategy.


 Please wait while we load your content...
Please wait while we load your content...