Late-stage diversification strategy for synthesizing ynamides through copper-catalyzed diynylation and azide–alkyne cycloaddition†
Abstract
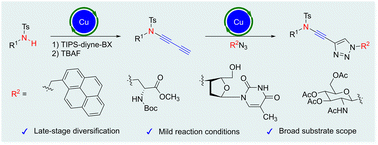
A late-stage diversification strategy for synthesizing ynamides has been developed. This strategy was enabled by the copper-catalyzed direct electrophilic diynylation of sulfonamides with a novel triisopropylsilyl diynyl benziodoxolone, deprotection, and the late-stage chemoselective copper-catalyzed azide–alkyne cycloaddition sequence, which yields various complex molecule-derived ynamides with pyrene, amino acid, nucleoside, and N-acetylglucosamine as substituents.


 Please wait while we load your content...
Please wait while we load your content...