Development of a novel sialic acid-conjugated camptothecin prodrug for enhanced cancer chemotherapy†
Abstract
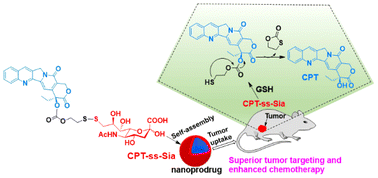
Camptothecin (CPT) is an attractive natural drug for cancer chemotherapy. However, the poor water solubility, non-targeting feature, and adverse side effects of CPT are significant obstacles to developing an effective anticancer drug. Here, for the first time, 9-thiol-sialic acid (9-SH-Sia) is coupled to CPT by forming a disulfide releasable carbonate linkage, resulting in a novel CPT prodrug (CPT-ss-Sia) that self-assembles into nanostructures in an aqueous solution. Strikingly, CPT-ss-Sia exhibited excellent in vitro properties, including enhanced water solubility, glutathione (GSH)-triggered CPT release, and increased E-lactone ring stability. Furthermore, CPT-ss-Sia had good cancer cell-killing ability comparable to CPT. Intravenous administration of CPT-ss-Sia significantly inhibited the growth of multiple types of tumors. Histological analysis showed that CPT-ss-Sia treatment significantly reduced lesions in tumor-bearing mice compared to CPT treatment. Notably, CPT-ss-Sia treatment did not adversely affect the body weight of the mice. This is the first report of the 9-SH-Sia conjugate-based prodrug. Overall, CPT-ss-Sia has broad clinical application prospects.


 Please wait while we load your content...
Please wait while we load your content...