Orthogonal electric and ionic conductivities in the thin film of a thiophene–thiophene block copolymer†
Abstract
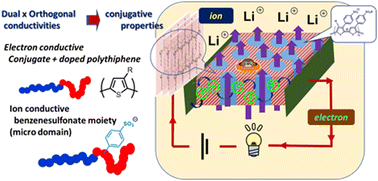
A thiophene–thiophene block copolymer composed of hydrophilic and hydrophobic side chain functionalities was designed and synthesized. The deprotonative metalation nickel-catalyzed polymerization protocol successfully afforded the block copolymer, in which the side chains are derived from alkyl and benzenesulfonic acid ester groups. The benzene sulfonate moiety of the block copolymer in the film state was shown to be transformed into hydrophilic sulfonic acid upon thermal treatment at ca. 200 °C without the addition of an external additive. Thus, the formed block copolymer thin film exhibited cylindrical microphase separation and the hydrophilic domain was revealed to penetrate the film perpendicular to the substrate. The measurement of electric conductivity suggested that the block copolymer thin film was conductive when placed parallel to the substrate, while the film was insulative when placed perpendicular to the substrate. Electrochemical analyses revealed that lithium ions transfer through the cylindrical domain composed of a benzene sulfonic acid side chain, which is perpendicular to the substrate. These results represent dual and orthogonal conductivities of electrons and ions in the block copolymer thin film.
- This article is part of the themed collection: #MyFirstJMCC


 Please wait while we load your content...
Please wait while we load your content...