A strong-alkali resistant zinc–organic framework with 1,3,6,8-tetra(pyridin-4-yl)pyrene for efficient photocatalytic hydrogen evolution†
Abstract
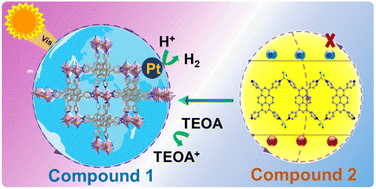
Photocatalytic hydrogen evolution using metal–organic frameworks (MOFs) has tremendous potential for relieving the environmental and energy crises. Herein, a three dimensional zinc–organic framework (1) was successfully synthesized based on 1,3,6,8-tetra(pyridin-4-yl)pyrene (TTPy). Compound 1 exhibited a good light absorption with an absorption edge at approximately 600 nm. Importantly, 1 can maintain the crystalline state in TEOA solution, and even remain stable in a 5 M NaOH aqueous solution, suggesting 1 has high alkali resistance. Photocatalytic results indicate that 1 as a photocatalyst has high activity for H2 production with TEOA as a sacrificial agent, and the H2 evolution rate is 315.06 μmol g−1 h−1 even under long reaction time (35 h) without the addition of photosensitizers. Mechanism explorations suggest that the π–π interaction between parallel ligands, high alkali-stability and light-absorbing ability, good photocurrent response, and suitable band gap of compound 1 are responsible for the high photocatalytic activity. Density functional theory (DFT) calculations further reveal that the experimental energy gap of 1 is well consistent with the theoretical bandgap. This work provides a new strategy for the exploitation of alkali-stable MOFs in the photocatalysis field.


 Please wait while we load your content...
Please wait while we load your content...