Adjusting the band gap of CsPbBr3−yXy (X = Cl, I) for optimal interfacial charge transfer and enhanced photocatalytic hydrogen generation†
Abstract
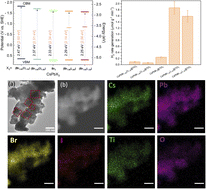
Metal halide perovskites (MHPs, CsPbX3: X = Cl, Br, I) demonstrate high photogenerated charge-carrier production and mobility, which makes them promising candidates for photocatalysis. In this work, we investigated how adjusting the band gap energy of MHPs at room temperature by anion exchange (CsPbBr3−yXy: X = Cl, Br, I) leads to optimal interfacial electron transfer from CsPbBr3−yXy to TiO2 by means of transient absorption spectroscopy (TAS), time-resolved photoluminescence (TRPL), and time-resolved microwave conductivity (TRMC). We found that the formation of indirect excitons at the MHPs/TiO2 interface results in slower charge-carrier relaxation, which is essential for photocatalysis. The substitution of bromide with chloride reduces the trapping states (healing effect), which favors charge-carrier relaxation to the ground state and leads to higher charge recombination and lower photocatalytic activity. The iodine, on its side, acts as a hole trapper proposing an optimal band gap facilitating fast charge injection in the TiO2. The charge-carrier injection from one material to another suppresses recombination, leading to impressive H2 generation.
- This article is part of the themed collection: Photofunctional Materials and Transformations


 Please wait while we load your content...
Please wait while we load your content...