A charge-free and membrane-free hybrid capacitive mixing system for salinity gradient energy harvesting†
Abstract
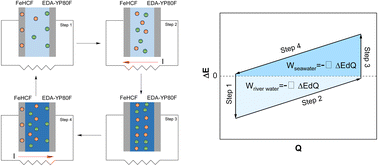
Capacitive mixing (CapMix) is a promising technology to harvest salinity gradient energy (SGE) by generating electricity through the potential difference owing to electrolyte salinity change at the electrode/electrolyte interface. However, existing CapMix methods suffer from the high cost of ion-exchange membranes, additional energy investment, and limited energy density and cycle life. Herein, we propose a charge-free and membrane-free hybrid CapMix system consisting of ethylenediamine-modified activated carbon (EDA-YP80F) as a capacitive anode and a Prussian blue analog, iron hexacyanoferrate (FeHCF), as a battery-type cathode. The selective incorporation/absorption of Na+ ions and Cl− ions into FeHCF and EDA-YP80F achieves a membrane-free operation. The matching potentials of FeHCF and EDA-YP80F enable reversible discharges to a final voltage of zero, eliminating the energy investment by external power sources. Without an external power source and membranes, the FeHCF/EDA-YP80F full cell achieves an average power density of 110 mW m−2 and energy density of 117 J m−2 over 150 cycles under the 1–0.01 M NaCl salinity gradient. Under a high salinity gradient involving hypersaline solutions, i.e., 5–0.01 M NaCl, a remarkably high average power density of 218 mW m−2 and energy density of 305 J m−2 are demonstrated over 50 cycles.


 Please wait while we load your content...
Please wait while we load your content...