Polystyrenes with both hydrophilic and hydrophobic moieties: synthesis and self-assembly behaviors†
Abstract
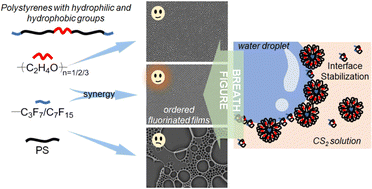
Fluorinated polymers are emerging as important alternatives for isoporous film fabrication by the breath figure technique, originating from the special characteristics endowed by fluorine elements such as low surface energy and good chemical stability. In this work, we design and synthesize polystyrenes (∼3600 Da) with perfluoroalkyl groups (–C3F7 or –C7F15) at both chain ends and hydrophilic oligo(ethylene glycol) units ((C2H4O)n, n = 1/2/3) in the chain middle by taking advantage of the bifunctional atom transfer radical polymerization (ATRP) initiators and post-substitution of the terminal bromine. We investigate the influence of the two disparate groups on the physical characteristics of the polymers and self-assembly behaviors during the dynamic breath figure process. It shows that the elongation of hydrophilic segments can greatly decrease the interfacial tension between the polymer solution and water (from 41.8 to 37.4 mN m−1), and the functionalization with perfluoroalkyl end groups diminishes the ability of the polymers to precipitate at the interface, as demonstrated by the cloud point results. Studies of the morphology of the porous films suggest that both low interfacial tension and strong capability of interfacial precipitation are beneficial to droplet stabilization and honeycomb pattern formation at low solution concentrations.


 Please wait while we load your content...
Please wait while we load your content...