Aminals as powerful XAT-reagents: activation of fluorinated alkyl chlorides†
Abstract
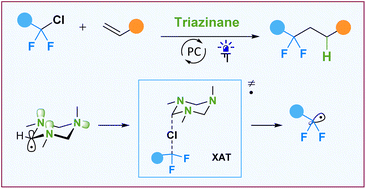
Readily available 1,3,5-trimethyl-1,3,5-triazinane serves as an efficient reagent for halogen atom transfer. Under photocatalytic conditions, the triazinane generates an α-aminoalkyl radical, which can activate the C–Cl bond of fluorinated alkyl chlorides. The hydrofluoroalkylation reaction between fluorinated alkyl chlorides and alkenes is described. The efficiency of the diamino-substituted radical derived from the triazinane is associated with stereoelectronic effects defined by a six-membered cycle forcing the anti-periplanar arrangement of the radical orbital and lone pairs of adjacent nitrogen atoms.


 Please wait while we load your content...
Please wait while we load your content...