Structure-guided semi-rational design of an imine reductase for enantio-complementary synthesis of pyrrolidinamine†
Abstract
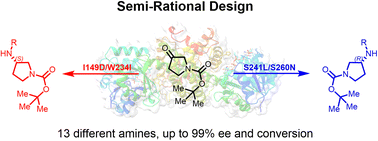
In this study, engineered imine reductases (IREDs) of IRED M5, originally from Actinoalloteichus hymeniacidonis, were obtained through structure-guided semi-rational design. By focusing on mutagenesis of the residues that directly interact with the ketone donor moiety, we identified two residues W234 and F260, playing essential roles in enhancing and reversing the stereoselectivity, respectively. Moreover, two completely enantio-complementary variants S241L/F260N (R-selectivity up to 99%) and I149D/W234I (S-selectivity up to 99%) were achieved. Both variants showed excellent stereoselectivity toward the tested substrates, offering valuable biocatalysts for synthesizing pyrrolidinamines. Its application was demonstrated in a short synthesis of the key intermediates of potential drug molecules leniolisib and JAK1 inhibitor 4, from cheap and commercially available pro-chiral N-Boc-piperidone 1 (2 and 3 steps, respectively).
- This article is part of the themed collection: 2023 Chemical Science Covers


 Please wait while we load your content...
Please wait while we load your content...