Geometry-dependent valence tautomerism, magnetism, and electrical conductivity in 1D iron–tetraoxolene chains†
Abstract
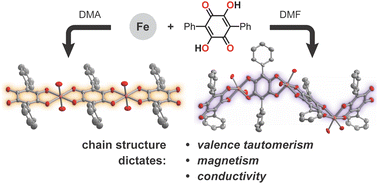
Redox-active tetraoxolene ligands such as 1,4-dihydroxybenzoquinone provide access to a diversity of metal–organic architectures, many of which display interesting magnetic behavior and high electrical conductivity. Here, we take a closer look at how structure dictates physical properties in a series of 1D iron–tetraoxolene chains. Using a diphenyl-derivatized tetraoxolene ligand (H2Ph2dhbq), we show that the steric profile of the coordinating solvent controls whether linear or helical chains are exclusively formed. Despite similar ligand environments, only the helical chain displays temperature-dependent valence tautomerism, switching from (FeII)(Ph2dhbq2−) to (FeIII)(Ph2dhbq3˙−) at temperatures below 203 K. The stabilization of ligand radicals leads to exceptionally strong magnetic exchange coupling (J = −230 ± 4 cm−1). Meanwhile, the linear chains are more amenable to oxidative doping, leading to Robin–Day class II/III mixed-valency and an increase in electrical conductivity by nearly three orders of magnitude. While previous studies have focused on the effects of changing metal and ligand identity, this work highlights how altering the metal–ligand connectivity can be a similarly powerful tool for tuning materials properties.


 Please wait while we load your content...
Please wait while we load your content...