Ni-catalyzed arylation of alkynes with organoboronic acids and aldehydes to access stereodefined allylic alcohols†
Abstract
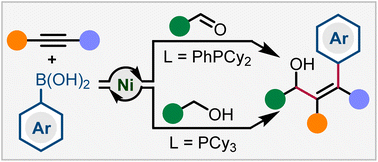
A new, efficient and practical method for the three-component arylative coupling of aldehydes, alkynes and arylboronic acids has been developed through nickel catalysis. This transformation provides diverse Z-selective tetrasubstituted allylic alcohols without the use of any aggressive oragnometallic nucleophiles or reductants. Moreover, benzylalcohols are viable coupling partners via oxidation state manipulation and arylative coupling in one single catalytic cycle. This reaction features a direct and flexible approach for the preparation of stereodefined arylated allylic alcohols with broad substrate scope under mild conditions. The utility of this protocol is demonstrated through the synthesis of diverse biologically active molecular derivatives.


 Please wait while we load your content...
Please wait while we load your content...