Hysteresis behavior in the unfolding/refolding processes of a protein trapped in metallo-cages†
Abstract
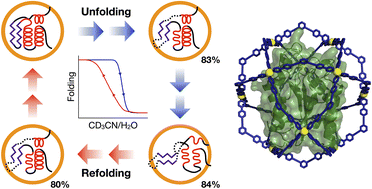
Confinement of molecules in a synthetic host can physically isolate even their unstable temporary structures, which has potential for application to protein transient structure analysis. Here we report the NMR snapshot observation of protein unfolding and refolding processes by confining a target protein in a self-assembled coordination cage. With increasing acetonitrile content in CD3CN/H2O media (50 to 90 vol%), the folding structure of a protein sharply denatured at 83 vol%, clearly revealing the regions of initial unfolding. Unfavorable aggregation of the protein leading to irreversible precipitation is completely prevented because of the spatial isolation of the single protein molecule in the cage. When the acetonitrile content reversed (84 to 70 vol%), the once-denatured protein started to regain its original folded structure at 80 vol%, showing that the protein folding/unfolding process can be referred to as a phase transition with hysteresis behavior.


 Please wait while we load your content...
Please wait while we load your content...