Drug repurposing screens identify compounds that inhibit α-synuclein oligomers' membrane disruption and block antibody interactions
Abstract
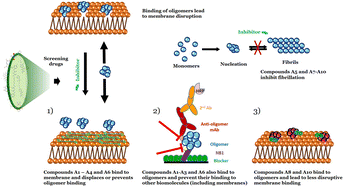
Small soluble oligomers of the protein α-synuclein (αSO) have been linked to disruptions in neuronal homeostasis, contributing to the development of Parkinson's Disease (PD). While this makes αSO an obvious drug target, the development of effective therapeutics against αSO is challenged by its low abundance and structural and morphological complexity. Here, we employ two different approaches to neutralize toxic interactions made by αSOs with different cellular components. First, we use available data to identify four neuronal proteins as likely candidates for αSO interactions, namely Cfl1, Uchl1, Sirt2 and SerRS. However, despite promising results when immobilized, all 4 proteins only bind weakly to αSO in solution in microfluidic assays, making them inappropriate for screening. In contrast, the formation of stable contacts formed between αSO and vesicles consisting of anionic lipids not only mimics a likely biological role of αSO but also provided a platform to screen two small molecule libraries for disruptors of these contacts. Of the 7 best leads obtained in this way, 2 significantly impaired αSO contacts with other proteins in a sandwich ELISA assay using αSO-binding monoclonal antibodies and nanobodies. In addition, 5 of these leads suppressed α-synuclein amyloid formation. Thus, a repurposing screening that directly targets a key culprit in PD pathogenesis shows therapeutic potential.
- This article is part of the themed collection: #MyFirstChemSci 2023


 Please wait while we load your content...
Please wait while we load your content...