A (TD-)DFT study on photo-NHC catalysis: photoenolization/Diels–Alder reaction of acid fluorides catalyzed by N-heterocyclic carbenes†
Abstract
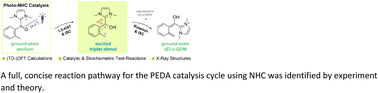
A comprehensive mechanistic study on the N-heterocyclic carbene (NHC) catalyzed photoenolization/Diels–Alder (PEDA) reaction of acid fluorides was performed in the framework of (time-dependent) density functional theory ((TD)-DFT). The 1,5-hydrogen atom transfer (1,5-HAT) during photoenolization of an ortho-toluoyl azolium salt was found to be feasible via, first, singlet excitation and photoenolization, and then, after crossing to the triplet manifold, populating a biradical dienol which allows for the formation of two ortho-quinodimethane (o-QDM) isomers due to a low rotational barrier. The (Z)-isomer is mostly unproductive through sigmatropic rearrangement back to the starting material while the (E)-isomer reacts in a subsequent concerted Diels–Alder reaction likely as the deprotonated dienolate. The experimentally observed diastereoselectivity is correctly predicted by theory and is determined by a more favorable endo trajectory in the cycloaddition step. These findings demonstrate that ortho-toluoyl azolium species exhibit similar photophysical properties as structurally related benzophenones, highlighting the unique ability of the NHC organocatalyst to transiently alter the excited state properties of an otherwise photoinactive carboxylic acid derivative, thereby expanding the scope of classical carbonyl photochemistry.


 Please wait while we load your content...
Please wait while we load your content...