Halohydrin dehalogenase-catalysed synthesis of enantiopure fluorinated building blocks: bottlenecks found and explained by applying a reaction engineering approach†
Abstract
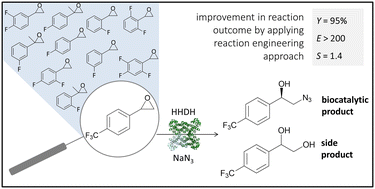
Optically pure fluorinated organic azides represent synthetically valuable building blocks in a range of industrial applications. Since direct fluorination of molecules is challenging from both economic and environmental perspectives, the development of novel methods for modifying existing fluorinated synthons is highly desirable. In this work, enantioselective azidolysis of fluorinated aromatic epoxides catalysed by halohydrin dehalogenases (HHDHs) was explored. A series of 11 fluorinated epoxides were evaluated as substrates from the viewpoint of hydrolytic stability and enzyme kinetics. The synthesis of enantiopure (R)-2-azido-1-[4-(trifluoromethyl)phenyl]ethanol with the HheC-W249P variant was selected for detailed kinetic investigation. Reaction bottlenecks were identified and discussed from the reaction engineering perspective. Epoxide hydrolysis, enzyme inhibition and operational stability decay were found to undesirably affect the reaction outcome. Understanding the kinetic limitations and applying model-based process simulations enabled the selection of the reactor type and initial conditions favouring biotransformation. High substrate loadings are not suitable since they support hydrolysis, enzyme deactivation, and substrate inhibition. By selecting a repetitive batch reactor set-up, a reaction yield of 95% could be obtained, together with the increase in the reaction selectivity of 100% compared to the batch reactor. To the best of our knowledge, the developed mathematical model represents so far the first of its kind with HHDH enzymes, thus bringing valuable insights into the kinetic and catalytic performance of this enzyme group, as well as the reaction type. It is expected that, with minor adaptations, it could be generalized and applied to give qualitative insight into the behaviour of similar systems.


 Please wait while we load your content...
Please wait while we load your content...