The role of nanocerianite (CeO2) in the stability of Ce carbonates at low-hydrothermal conditions†
Abstract
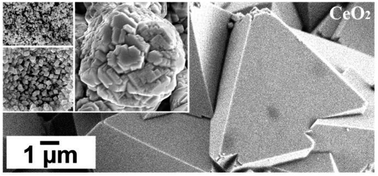
The formation of cerianite (CeO2) was investigated at low hydrothermal conditions (35–205 °C) via two experimental settings: (1) crystallisation from solution experiments, and (2) replacement of Ca–Mg carbonates (calcite, dolomite, aragonite) mediated by Ce-bearing aqueous solutions. The solid samples were studied with a combination of powder X-ray diffraction, scanning electron microscopy, and Fourier-transform infrared spectroscopy. The results revealed a multi-step crystallisation pathway: amorphous Ce carbonate → Ce-lanthanite [Ce2(CO3)3·8H2O] → Ce-kozoite [orthorhombic CeCO3(OH)] → Ce-hydroxylbastnasite [hexagonal CeCO3(OH)] → cerianite [CeO2]. We found that Ce carbonates can decarbonise in the final stage of the reaction, forming cerianite which significantly increases the porosity of the solids. The redox behaviour of Ce combined with the temperature, and the availability of CO23− govern this crystallisation sequence, the sizes, morphologies, and crystallisation mechanisms of the solid phases. Our results explain the occurrence and behaviour of cerianite in natural deposits. These findings also present a simple, environmental-friendly, and cost-efficient method for the synthesis of Ce carbonates and cerianite with tailored structures and chemistries.


 Please wait while we load your content...
Please wait while we load your content...