Regioselective electrophilic aromatic borylation as a method for synthesising sterically hindered benzothiadiazole fluorophores†
Abstract
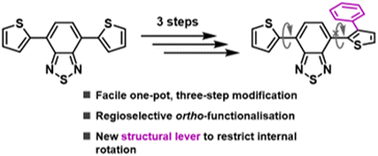
Regioselective stepwise phenylation of 4,7-diarylbenzo[c][1,2,5]thiadiazole fluorophores has been achieved through a facile one-pot, three-step synthetic strategy involving sequential borylation, hydroxydechlorination and Suzuki–Miyaura cross-coupling reactions. Crucial to the selectivity was the use of BCl3 to regioselectively install a boronic acid group in the ortho-position of only one of the diaryl groups. The subsequent introduction of ortho-phenyl groups through Suzuki–Miyaura cross-coupling gave rise to twisted structures with hindered intramolecular rotation, providing a structural lever with which the fluorophore absorption and emission properties could be adjusted.


 Please wait while we load your content...
Please wait while we load your content...