Anion exchange on hydrous zirconium oxide materials: application for selective iodate removal†
Abstract
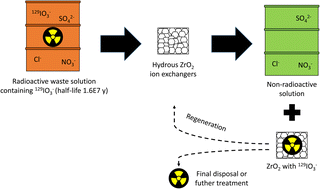
The radioactive 129I is a top-priority radionuclide due to its the long half-life (1.57 × 107 years) and high mobility. Because of the planned and accidental releases to the environment, specific separation technologies are required to limit the potential radiation dose to human beings. Zirconium oxides are known for their adsorption capability and selectivity to oxyanions and here the applicability to selective IO3− removal has been investigated regarding the uptake mechanism, regeneration and competition caused by other anions, like environmentally relevant SO42−. Granular aggregates of hydrous zirconium oxides with and without Sb doping showed high potential for the selective IO3− removal in the presence of competing anions, like the forementioned SO42− (apparent capacity between 0.1–0.4 meq g−1 depending on SO42− concentration). The main uptake mechanism was found to be outer-sphere complexation (ion-exchange) to the protonated hydroxyl groups of hydrous zirconium oxides, but also minor mechanisms were identified including inner-sphere complexation and reduction to I−. The materials were observed to be easily and successively regenerated using dilute acid. Hydrous zirconium oxides showed high potential for IO3− removal from waste solutions regarding technical (high selectivity and apparent capacity) and ecological/economic (feasible regeneration) aspects.
- This article is part of the themed collection: RSC Advances Outstanding Student Paper Awards 2023


 Please wait while we load your content...
Please wait while we load your content...