Abstract
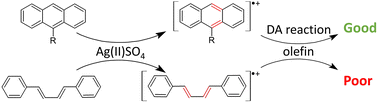
This work presents the joint experimental and computational analysis of Diels–Alder (DA) reactions initiated by one-electron oxidation of dienes: anthracene and buta-1,3-diene derivatives. The use of AgSO4 – a very strong one-electron oxidizer – results in reactions proceeding via radical cationic intermediates rather than neutral species. In the case of anthracenes, yields of reactions are significantly enhanced compared to those for their neutral variants. Importantly, this includes also 9-halogenated anthracenes for which DA reactions reported in the literature are scarce. Surprisingly, diene oxidation has only a limited effect on derivatives of buta-1,3-diene. DFT modelling indicates that radical cations of dienes undergo DA reactions with dienophiles more readily than the corresponding neutral species. For anthracene derivatives, calculated barriers are in qualitative agreement with experimental findings. Careful analysis of the whole catalytic cycle allows us to point out various bottlenecks which are responsible for the poor performance of diene oxidation in reactions involving butadienes.


 Please wait while we load your content...
Please wait while we load your content...