C(sp3)–H fluorosulfonylvinylation/aza-Michael addition approach to FSO2-functionalized tetrahydropyridines†
Abstract
A novel cascade approach is presented for the synthesis of FSO2-functionalized tetrahydropyridines from propargyl alcohols, FSO2Cl, and anilines. This strategy successively involves radical fluorosulfonylation of the alkyne, C(sp3)–H fluorosulfonylvinylation, enamine formation, and intramolecular aza-Michael addition. Notably, the fluorosulfonyl radical can be generated efficiently via simple blue light irradiation of an electron donor–acceptor (EDA) complex between propargyl alcohols and FSO2Cl, which requires no base, catalyst, and additive. The versatile follow-up derivatizations allow rapid ligation of tetrahydropyridines with other bioactive molecules that will be of value for drug discovery.
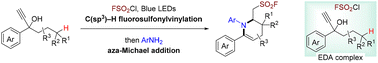


 Please wait while we load your content...
Please wait while we load your content...