Disulfides as versatile starting reagents: effective sulfonylation of alkenes with disulfides under electrochemical conditions†
Abstract
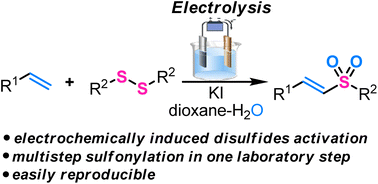
Electrochemically induced sulfonylation of alkenes with disulfides as the starting reagents is developed. This transformation is a quite rare example of disulfides usage as S-partners in electrochemical C–S coupling. In many cases, disulfides are inert in electrochemical coupling, and their formation in reactions starting from thiols (their synthetic precursors) is a dead-end pathway. In the discovered process, vinyl sulfones are formed exclusively, which is surprising. Previously, in reactions involving disulfides, only sulfenylation took place, resulting in sulfides, which could be transformed into the corresponding sulfones only through oxygenation with mCBPA or other similar oxygen donors. The developed reaction is carried out under constant current conditions in an experimentally convenient undivided electrochemical cell equipped with a platinum anode and a stainless-steel cathode. KI acts in this process as a supporting electrolyte and redox catalyst, which enables the formation of sulfonylating species from the starting disulfides. Taking into account the results of control experiments, a CV study, and literature data, we propose that both radical and ionic pathways could be involved in the formation of the desired products.


 Please wait while we load your content...
Please wait while we load your content...